Den pharmaceutical industry operates under a unique set of constraints, where precision and purity are paramount. Every stage of pharmaceutical production, from the initial formulation of a drug to its final packaging, demands meticulous attention to detail to ensure both the safety and efficacy of the end product. While often overlooked, atomization equipment plays a crucial, yet often unseen, role in this intricate world. From creating perfectly sized drug particles with predictable dissolution rates to coating tablets for optimal drug release and stability, atomization technology acts as a silent hero in pharmaceutical manufacturing. This in-depth exploration delves into the fascinating applications of atomization equipment in this demanding field, examining the key techniques, their benefits, and the sophisticated equipment that makes it all possible.

Why Atomization Matters in Pharmaceutical Manufacturing
Imagine trying to evenly distribute a pinch of salt across an entire football field. That’s the level of precision and control required in pharmaceutical manufacturing, where even the slightest variations in particle size or coating thickness can have significant consequences on drug efficacy, bioavailability, and patient safety. Here’s a closer look at why atomization is not just beneficial, but indispensable:
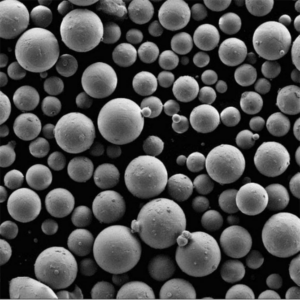
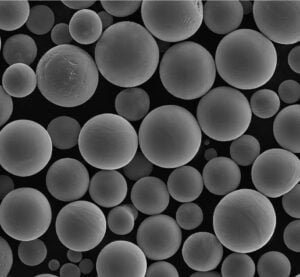
- Precise Particle Size Control: The size of drug particles directly impacts their dissolution rate, bioavailability, and overall effectiveness. Atomization allows manufacturers to create particles within a very narrow size range, ensuring consistent drug delivery and therapeutic outcomes. This is particularly critical for poorly soluble drugs, where particle size reduction through techniques like micronization or nanonization can dramatically improve bioavailability, leading to lower dosages and potentially fewer side effects.
- Enhanced Drug Delivery: Atomization enables the creation of micro and nano-sized drug particles, opening up possibilities for targeted drug delivery systems like liposomes, nanoparticles, solid lipid nanoparticles, and microspheres. These tiny particles can penetrate cell membranes more effectively, improving drug absorption, targeting specific tissues or cells, and reducing side effects by minimizing off-target drug deposition. This targeted approach is particularly valuable for treating diseases like cancer, where minimizing drug exposure to healthy tissues is crucial.
- Uniform Coating Application: Tablets and capsules often require coatings to mask taste, control drug release, or protect the active ingredient from degradation. Atomization ensures uniform coating application, resulting in consistent product quality, improved stability, and enhanced patient compliance. For instance, enteric coatings applied via atomization protect acid-labile drugs from the harsh environment of the stomach, ensuring release in the intestine for optimal absorption. Controlled-release coatings, on the other hand, can be precisely engineered to deliver medication over extended periods, improving patient adherence and therapeutic outcomes.
- Increased Production Efficiency: Atomization processes are highly efficient, allowing for large-scale production of pharmaceuticals while maintaining stringent quality standards. This efficiency translates to cost savings throughout the manufacturing process, from reduced material waste to faster production times. This ultimately benefits patients by increasing the accessibility and affordability of life-saving medications.
Key Atomization Techniques in Pharmaceutical Manufacturing
Just as a skilled chef uses different knives for specific culinary tasks, pharmaceutical manufacturers rely on a diverse array of atomization techniques to achieve desired outcomes in drug formulation and production. Each technique offers unique advantages and is tailored to specific applications and material properties. Let’s explore some of the most prevalent methods:
1. Spray Drying: A Cornerstone of Pharmaceutical Production
Spray drying is a widely used atomization technique in pharmaceutical manufacturing, particularly for transforming liquid feeds into solid powders. This process involves atomizing a liquid feed (containing the active pharmaceutical ingredient, excipients, and often dissolved polymers) into a fine spray of droplets within a drying chamber. Hot gas, typically air, flows through the chamber, rapidly evaporating the solvent from the droplets and leaving behind solid particles. The rapid drying process minimizes heat exposure to the active ingredient, making it suitable for heat-sensitive drugs. This technique is particularly valuable for producing dry powder inhalers, where precise particle size control is essential for efficient drug delivery to the lungs, and for creating amorphous solid dispersions, which enhance the solubility and bioavailability of poorly soluble drugs.
| Funktion | Beskrivning | Fördelar |
|---|---|---|
| Process | Atomization of liquid feed into a hot drying chamber | Efficient, scalable, suitable for heat-sensitive materials |
| Partikelstorlek | Typically in the micrometer range, but can be tailored to nanometer size with specialized equipment | Controllable particle size distribution for optimal drug delivery |
| Tillämpningar | Production of powders for tablets, capsules, inhalers, injectables, and amorphous solid dispersions | Versatile technique for a wide range of pharmaceutical products |
2. Fluid Bed Processing: Versatility in Drying and Coating
Fluid bed processing encompasses a range of techniques that utilize a fluidized bed of particles. In the context of atomization, fluid bed processors are commonly used for drying granules, coating particles, or building layers of different materials onto a core particle. The process involves suspending particles in an upward-flowing stream of air, creating a fluidized state where the particles behave like a fluid. A liquid solution containing the coating material or drying gas is then introduced into the fluidized bed, where it interacts with the particles as the solvent evaporates or heat is transferred. This technique offers excellent control over coating thickness and uniformity, making it ideal for applications like controlled-release drug formulations, taste masking, and functional coating applications.
Key Applications:
- Tablet Coating: Applying functional coatings to tablets for taste masking, controlled release, or enteric protection. This ensures that the drug is released at the desired rate and location within the digestive system, optimizing its therapeutic effect.
- Granulation: Creating granules from fine powders to improve flow properties and compressibility for tablet manufacturing. This step is crucial for ensuring consistent tablet weight, hardness, and disintegration properties, which ultimately impact the drug’s release profile.
- Powder Layering: Applying multiple layers of different materials onto particles for controlled drug release profiles or to combine incompatible ingredients. This allows for the creation of sophisticated drug delivery systems that release medication over extended periods, improve drug stability, or target specific areas of the body.
3. Supercritical Fluid Technology: A Greener Approach
Supercritical fluid technology is an emerging atomization technique gaining traction in pharmaceutical manufacturing due to its environmental friendliness and ability to process sensitive materials, including proteins, peptides, and polymers. This method utilizes a supercritical fluid, typically carbon dioxide (CO2), as the solvent. Supercritical fluids exhibit properties of both liquids and gases, allowing them to dissolve and transport materials like a liquid while maintaining the low viscosity of a gas. This unique combination of properties allows for the production of very fine particles with narrow size distributions and controlled morphology, making it ideal for creating nanoparticle drug delivery systems, enhancing drug solubility, and developing novel drug formulations.
Fördelar:
- Gentle Processing: Ideal for heat-sensitive drugs and biologics, as the process operates at relatively low temperatures, preserving the integrity of sensitive molecules and minimizing degradation.
- Solvent-Free Products: The supercritical fluid, often CO2, can be easily removed from the final product by simply reducing the pressure, leaving behind no harmful solvent residues. This makes it ideal for pharmaceutical applications where purity is paramount, particularly for injectable and inhaled drug products.
- Kontroll av partikelstorlek: Allows for precise control over particle size and morphology, enabling the creation of nanoparticles and microparticles with tailored properties for targeted drug delivery, improved bioavailability, and controlled release applications.
Essential Atomization Equipment for Pharmaceutical Manufacturing
Achieving precise and controlled atomization in pharmaceutical manufacturing requires specialized equipment designed to meet the stringent demands of the industry, ensuring product quality, safety, and regulatory compliance. These systems are engineered for precision, reliability, and often operate under strict cleanroom conditions to prevent contamination. Here are some key equipment categories:
1. Atomizers and Nozzles: The Heart of the Process
Atomizers and nozzles are the core components responsible for generating the fine droplets or particles required in various pharmaceutical processes. They come in a variety of designs, each optimized for specific applications and materials, from low viscosity solutions to highly viscous suspensions. The selection of the right atomizer or nozzle depends on factors like the desired droplet or particle size, flow rate, material properties, and the specific application.
- Two-Fluid Nozzles: These nozzles use compressed gas, typically air or nitrogen, to atomize a liquid feed. They are versatile and widely used in spray drying and fluid bed processing, offering control over droplet size by adjusting gas and liquid flow rates. The atomization mechanism involves the high-velocity gas stream shearing the liquid into droplets, with smaller droplets achieved at higher gas velocities and lower liquid flow rates.
- Ultrasonic Nozzles: These nozzles utilize high-frequency vibrations, typically generated by a piezoelectric transducer, to atomize the liquid feed. They are particularly suitable for applications requiring low flow rates and small droplet sizes, such as the production of liposomes or nanoparticles for drug delivery, as well as coating applications where precise and uniform deposition is critical.
- Rotary Atomizers: These atomizers use a rapidly spinning disc or wheel to atomize the liquid feed. They are known for their high throughput and ability to handle viscous materials, making them suitable for large-scale pharmaceutical production. The atomization mechanism involves centrifugal force flinging the liquid off the rotating disc in the form of droplets, with droplet size controlled by the rotational speed and the properties of the liquid.
2. Spray Dryers: From Liquid to Powder
Spray dryers are complete systems designed to transform liquid feeds into dry powders using atomization and controlled drying. They typically consist of an atomizer, a drying chamber, a heating system, a powder collection system, and a control system to manage process parameters. These systems are highly customizable, allowing manufacturers to optimize parameters like temperature, flow rate, drying time, and atomization gas pressure to achieve the desired particle size, morphology, and powder properties. Spray dryers are essential for manufacturing a wide range of pharmaceutical products, including dry powder inhalers, tablets, capsules, and powdered intermediates for further processing.
3. Fluid Bed Processors: Multi-Purpose Platforms
Fluid bed processors are versatile systems used for a range of particle processing applications, including drying, granulation, and coating. They typically consist of a processing chamber, an air handling unit, a heating or cooling system, a filter system for exhaust air treatment, and a control system. These systems offer precise control over process parameters like temperature, air flow, and coating solution application rate, ensuring consistent product quality. Fluid bed processors are commonly used in the pharmaceutical industry for operations like drying of granules, coating of tablets for taste masking or controlled release, and granulation of powders to improve flow properties for tablet compression.
4. Supercritical Fluid Equipment: Precision and Purity
Supercritical fluid equipment encompasses a range of systems designed for processes like particle formation, extraction, and cleaning. These systems typically include a CO2 pump, a pressure vessel, a temperature control system, specialized nozzles for supercritical fluid atomization, and a product collection system. This equipment enables the production of highly pure particles with controlled size and morphology, making it ideal for advanced drug delivery systems, developing novel drug formulations with enhanced solubility, and extracting valuable compounds from natural sources.
The Future of Atomization in Pharmaceutical Manufacturing
The pharmaceutical industry is continuously evolving, driven by the pursuit of more effective, targeted, and patient-centric drug delivery solutions, as well as the need for more efficient and sustainable manufacturing processes. Atomization technology is poised to play an even more significant role in this evolution, with ongoing advancements focused on:
- Personanpassad medicin: Atomization enables the creation of customized drug formulations tailored to individual patient needs, paving the way for personalized medicine. This includes the development of patient-specific dosages, drug combinations, and delivery systems based on individual genetic and physiological factors, leading to more effective treatments with fewer side effects.
- Nanomedicine: The ability to create nanoparticles and nanostructured materials using atomization is driving innovation in nanomedicine, enabling targeted drug delivery and improved therapeutic outcomes. This includes the development of nanoparticles that can cross the blood-brain barrier for targeted delivery of drugs to the brain, as well as nanoparticles that can target specific cancer cells, minimizing side effects on healthy tissues and improving treatment efficacy.
- Continuous Manufacturing: Atomization is a key enabling technology for continuous manufacturing processes, which offer increased efficiency, reduced waste, and enhanced process control in pharmaceutical production. This shift from batch processing to continuous flow systems allows for real-time monitoring and adjustment of process parameters, ensuring consistent product quality, reducing production time and costs, and enabling more agile and responsive manufacturing processes.
FAQs: Delving Deeper into Atomization in Pharmaceutical Manufacturing
1. What are the main advantages of using atomization techniques in drug formulation compared to traditional methods?
Atomization offers several key advantages over traditional methods, including:
- Precise particle size control: This leads to consistent drug release, improved bioavailability, and potentially lower dosages.
- Enhanced drug delivery: Creating micro and nano-sized particles enables targeted delivery, reducing side effects and improving therapeutic efficacy.
- Uniform coating application: Ensures consistent product quality, controlled drug release, taste masking, and improved stability.
- Increased production efficiency: Large-scale production with stringent quality control leads to cost savings and increased accessibility of medications.
2. Can you explain the difference between spray drying and fluid bed processing in simple terms?
Both techniques involve atomization but differ in their applications:
- Spray drying: Transforms liquids into dry powders. It’s ideal for heat-sensitive materials and creating inhalable drug formulations.
- Fluid bed processing: Used for drying granules, coating particles, or layering materials. It’s versatile for controlled release and functional coatings.
3. Why is supercritical fluid technology considered a greener approach in pharmaceutical manufacturing?
Supercritical fluid technology, primarily using CO2, offers several environmental benefits:
- Gentle processing: Uses low temperatures, making it ideal for heat-sensitive drugs and minimizing degradation.
- Solvent-free products: CO2 is easily removed, leaving no harmful residues, which is crucial for pharmaceutical purity.
4. What types of equipment are essential for carrying out atomization processes in pharmaceutical production?
Several types of equipment are crucial, each with specific functions:
- Atomizers and nozzles: Generate the fine droplets or particles, with different designs for various applications and materials.
- Spray dryers: Transform liquid feeds into dry powders, essential for inhalers and other powder formulations.
- Fluid bed processors: Versatile systems for drying, granulation, and coating, ensuring consistent product quality.
- Supercritical fluid equipment: Enables the production of highly pure particles with controlled size and morphology.
5. How is atomization technology contributing to advancements in personalized medicine and nanomedicine?
Atomization plays a key role in these emerging fields:
- Personalized medicine: Enables customized drug formulations tailored to individual patient needs, leading to more effective and personalized treatments.
- Nanomedicine: Facilitates the creation of nanoparticles for targeted drug delivery, improving therapeutic outcomes and minimizing side effects.
Conclusion: Embracing the Power of Tiny Droplets
Atomization equipment, though often operating behind the scenes, plays a vital role in ensuring the precision, purity, and effectiveness of pharmaceutical products. From creating perfectly sized drug particles to applying uniform coatings, atomization techniques are essential for achieving desired drug release profiles, improving bioavailability, enhancing patient compliance, and enabling the development of novel drug delivery systems. As the industry continues to advance, embracing the power of atomization will be crucial for developing innovative drug delivery solutions, improving the efficiency and sustainability of pharmaceutical manufacturing, and ultimately, meeting the evolving needs of patients worldwide.